Publishing is the lifeblood of science
I love writing papers.
I really enjoy the combination of creativity and logic required plus the sense of drawing a line under a piece of work. It’s also great (usually!) to get feedback from the scientific community both during and after the peer review process. I rarely have anyone disagree with me on this, yet it would seem we sit on unpublished work that could inform the scientific community and positively influence careers and institutional reputations.
So what’s stopping you?
There is no perfect formula to get from a ‘stack of data’ to a publication but here are five of my tips to help you get started on that next paper:
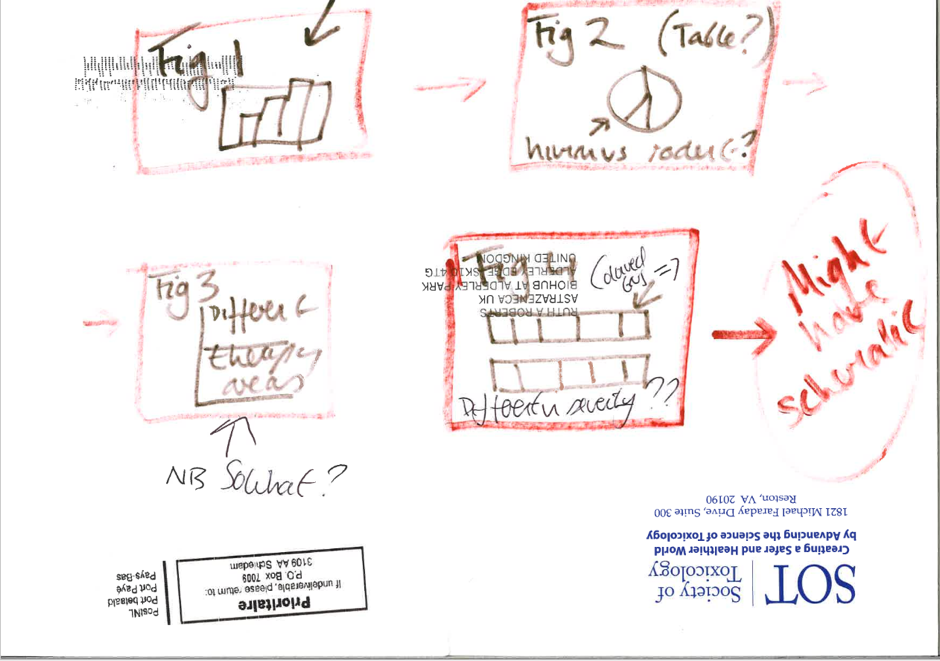
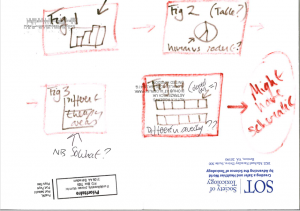
Make a storyboard
It’s easy to get lost in details so start with a ‘rough sketch’ storyboard – what are the key points or messages and how do I assemble them into a logical order? Use a ‘straw man’ to get the creativity going and be controversial to engage your team in the discussion. The storyboard may be nothing more than a sketched-out draft of the results section so don’t bother with fancy diagrams and detailed tables until you have the message right. Be prepared to ditch data sets that don’t add anything!
Create a team
This could be obvious – you may already have a team of people who contributed to the direction, designed the study and generated the data. However, consider bringing a relative outsider (eg a peer or colleague) into the draft and review process to provide challenge and constructive criticism. Now try your storyboard out on them. Give them a clear brief: does it hang together? What’s missing? What’s superfluous?
Be clear on authorship as early as possible
Not just who, but rather a clear understanding of what is expected of an author on your paper. You might be the first or last author depending on your role but you need a clear idea of who is included and why. Writing a paper together is a positive team-building experience but it can be ruined by unrealistic expectations. Don’t reinvent the wheel on this – the ICMJE guidelines set out roles and responsibilities – read and share these around as early as possible!
Write in the right order
Once you have your storyboard agreed with the team, draft the results section. Then tackle the methods section making sure it’s entirely consistent with the results you have included and nothing extra. Now write the discussion – this usually helps to spot gaps in the logic flow so don’t be put off if you feel you need to go back to the storyboard to align the key points. Now draft the introduction to set the scene for the hypothesis you are testing, why it’s important and what was the approach used to test it? In essence, this is the ‘tell me why I should care’ part of the paper. And finally, when the paper really feels like it’s come together, write the abstract and title…..
Find the time
A good basic outline or storyboard can be a great motivational aid to help you find the time to get going with that first elusive draft. Alternatively, look through your schedule, ring-fence some time and be disciplined with yourself! Most of my papers were written at thirty-five thousand feet over the Atlantic. This is a great example of finding >6 hours of uninterrupted thinking time to get to grips with your task. So turn off the film and get working…
The first story board for one of my papers…..and yes this is the back of an envelope.