
Verve halts enrollment of lead trial after grade 3 side effects, prioritizing next-up PCSK9 editor
By Max Bayer
VERVE-101 is a gene editing treatment for serious high cholesterol which was recently tested in 6 patients in the early stages of a phase 1 study. The study was halted due to grade 3 increases in a particular liver enzyme and a grade 3 case of low blood platelets in one of the patients. The new VERVE-102 lipid nanoparticle provides alternative pathways of entry into the liver. Trials of VERVE-102 will begin dosing this year.
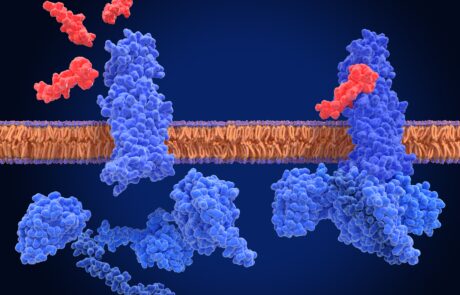
Study finds no link between Novo Nordisk’s GLP-1 drugs and suicidal ideation
By Kevin Dunleavy
Novo Nordisk’s recent GLP-1 drugs for diabetes and obesity including Ozempic, have been monitored by the FDA for side effects including suicidal ideation. A study of medical records of patients in the United States shows no link between use of the GLP-1 drugs and an increase in suicidal thoughts. Semaglutide was found to be less likely to cause suicidal thoughts in patients compared to patients on other diabetes and obesity treatments.

Gold Nanoparticles (AuNPs)-Toxicity, Safety and Green Synthesis: A Critical Review
By Niznik et al
AuNPs have a wide variety of uses, including the medicinal field for drug delivery systems, diagnostics, and therapeutic agents. This article comprehensively reviews recent advancements in AuNPs research, encompassing synthesis methodologies, diverse applications, and crucial insights into their toxicological profiles.

Tacrolimus-Induced Neurotoxicity After Transplant: A Literature Review
By Verona et al
Immunosuppressants to prevent rejection after organ transplantation such as the calcineurin inhibitor, Tacrolimus, are commonly associated with various toxicities. This comprehensive review may guide further studies to investigate the pathophysiology of tacrolimus-induced neurotoxicity and to define patient-specific strategies for mitigation or minimization of neurotoxicity.

Drug-Induced Liver Injury in the Elderly: Consensus Statements and Recommendations from the IQ-DILI Initiative
By Cohen et al
The number of older people in the population is projected to exceed 1.5 billion people by 2050 and so the prevalence of drug-induced liver injury (DILI) and other safety events are also likely to increase. DILI due to specific drugs might be more common in this population. Improved characterization of DILI in the elderly may enhance diagnostic and prognostic capabilities and improve the way in which liver safety is monitored during clinical trials.

Promoting Collaboration of Regulators and Patients in Improving Drug Safety and Regulatory Decision Making
By Khoo et al
Although patients are the end users of medicines and experts in their medical conditions, several key challenges currently hinder systemic patient involvement in drug safety and regulatory decision making. Addressing the challenges detailed in this article will greatly advance collaboration among regulators, patients, and patient advocates to enhance drug safety and regulatory decision making.

The synthetic cannabinoids menace: a review of health risks and toxicity
By Alzu’bi et al
Synthetic cannabinoids can initiate pathophysiological changes in many tissues which can be severe enough to damage the normal functionality. This review investigates the multisystem complications found in synthetic cannabinoid abusers, particularly discussing their neurologic, cardiovascular, renal, and hepatic effects.
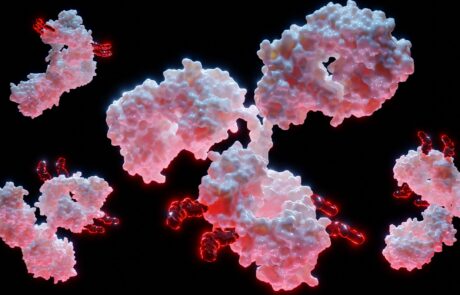
Merck & Co. signs $208M deal to acquire start-up in bid to improve ADC safety
By James Waldron
Antibody-drug conjugates (ADCs) class of drugs designed to target cancers by selectively killing tumour cells while leaving healthy cells unharmed. The University at Buffalo startup, Abceutics, was recently taken over by Merck & Co to expand its ADC pipeline.
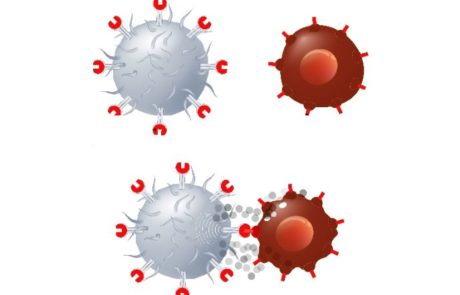
FDA flags early deaths in CAR-T myeloma trials for J&J and Legend’s Carvykti, Bristol Myers’ Abecma
By Angus Liu
Concerns have been raised by the FDA around the CAR-T trials of Carvykti and Abecma due to increased rates of death due to adverse events. Experts in the field are due to discuss whether the negative trend is “acceptable in the context of the clinical benefit”, to aid decision making in approval of the compounds for clinical use.
