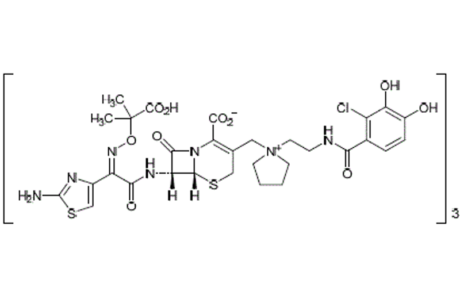
FDA approves Shionogi’s antibiotic Fetroja with spooky mortality warning label
By Kyle Blankenship
Fetroja is the latest antibiotic produced by Shinogi has just been approved by the FDA, despite 33.7% mortality rate for patients treated with Fetroja at the 49-day mark. In the face of this, the approval of this drug was due to data from phase III showing that the primary endpoint for Fetroja was met by 72.6% of patients after a week.
Novartis gene therapy ambitions dealt another blow by FDA hold on Zolgensma
By Jonathan Gardner
Novartis’ gene therapy trial, STRONG, aims to treat spinal muscular atrophy in older children by intrathecal infusion of their Zolgensema drug. Despite prior scandal with their preclinical data, a partial hold has been placed on the STRONG trial due to a paper publication of a small animal study revealing possible inflammation of dorsal root ganglia sensory neurons.
Pfizer’s Xeljanz gets EMA blood clots warning following FDA’s similar move
By Angus Liu
The European Medicines Agency announced that Pfizer’s immunology drug, Xeljanz, and its 10mg twice-daily dosing regime should only be used as a last resort for ulcerative colitis, if no other treatment is available. This comes with a FDA boxed warning of serious infections due to data from a post marketing study in rheumatoid arthritis patients, which indicated dangerous cardiovascular signals and increased risk of blood clots in leg veins and lungs.
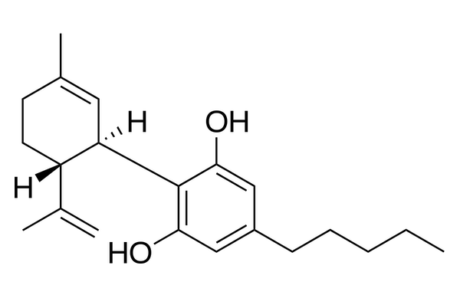
FDA warns 15 companies for illegally selling various products containing cannabidiol as agency details safety concerns
By FDA
Letters have been sent by the FDA to 15 different companies warning them of the ways in which they are illegally selling products containing cannabidiol (CBD). Violations include marketing unapproved new human and animal drugs, selling CBD products as dietary supplements, and adding CBD to human, and animal foods. The FDA cannot confirm that CBD is generally recognised as safe when used in these circumstances.
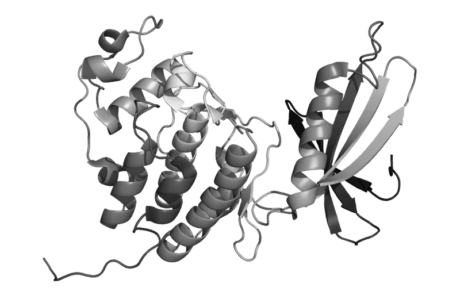
Strategies for Early Prediction and Timely Recognition of Drug-Induced Liver Injury: The Case of Cyclin-Dependent Kinase 4/6 Inhibitors
By E Raschi and F De Ponti
The relevance of investigating post-marketing drug-induced liver injury (DILI) is exemplified by the vast range of agents which induce it; dietary supplements; medications; herbal remedies. Several mechanisms of predicting and recognising DILI are being developed such as using novel oral anticancer medications, cyclin-dependent kinase 4/6 inhibitors as well as unpublished post-marketing reports.
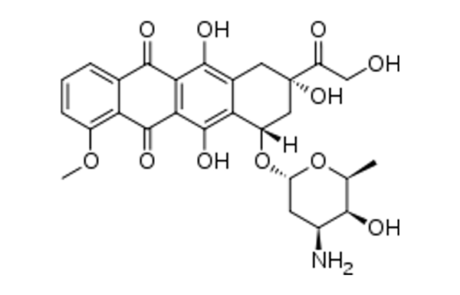
Mechanisms and clinical course of cardiovascular toxicity of cancer treatment I. Oncology.
By ETH Yeh et al
Cardiovascular toxicity remains a major safety concern for oncology drugs due to their link to heart failure. Speculation arises bases on extensive studies on anthracycline cardiotoxicity and its unusual differences between toxicity mechanism and oncologic efficacy. Better understanding of β-topoisomerase II, dexrazoxane and several tyrosine-kinase inhibitors advanced knowledge of the cascade of events leading to heart failure.

AHA: Johnson & Johnson, Bayer still looking for answers in aborted Xarelto study
By Kyle Blankenship
Johnson & Johnson and Bayer prematurely ended their comparative study of Xarelto last year due to “imbalanced” data regarding the safety of Xarelto. The drug was being used as an anti-coagulent in patients who had a transcatheter aortic valve replacement. Although, Xarelto showed higher chance of bleeding and thrombotic events. The unusual data is still not understood and that ending the study was precautionary, in the light of patient death.
Solid gene therapy trial halted again by FDA
By Jonathan Gardner
The clinical trial of Solid Biosciences’ gene therapy drug SGT- 001 to treat Duchenne muscular dystrophy has been halted by the FDA. This is due to complications in a patient experiencing the highest dose (200 trillion vector genomes per kilogram of bogy weight) of SGT-001. Complications include; complement activation; kidney damage; cardio-pulmonary insufficiency; a drop in red blood cell and platelet counts.

Muscle Toxicity of Drugs: When Medication Turns Physiology into Pathophysiology
By Janssen Lando et al
When treating diseases with new drug compounds in man, muscle toxicity is a common but often unexpected drug-derived symptom. Although these myotoxicities are variable based on the drug and from patient- to- patient, there is a clinical need to develop a method of testing compounds against this myotoxicity.
