Seizure Liability
The regulations regarding the testing of new drugs are changing. The FDA modernisation act endorses the use of new approach methodologies (NAMs) to assess of a range of toxicities, including seizure liability which can negatively impact a drug’s probability of success. We have developed an integrated in vitro screening approach for early seizure liability to support hazard identification and decision making in early drug discovery:
- A panel of 15 human ion channels related to seizure that can be screened using automated electrophysiology
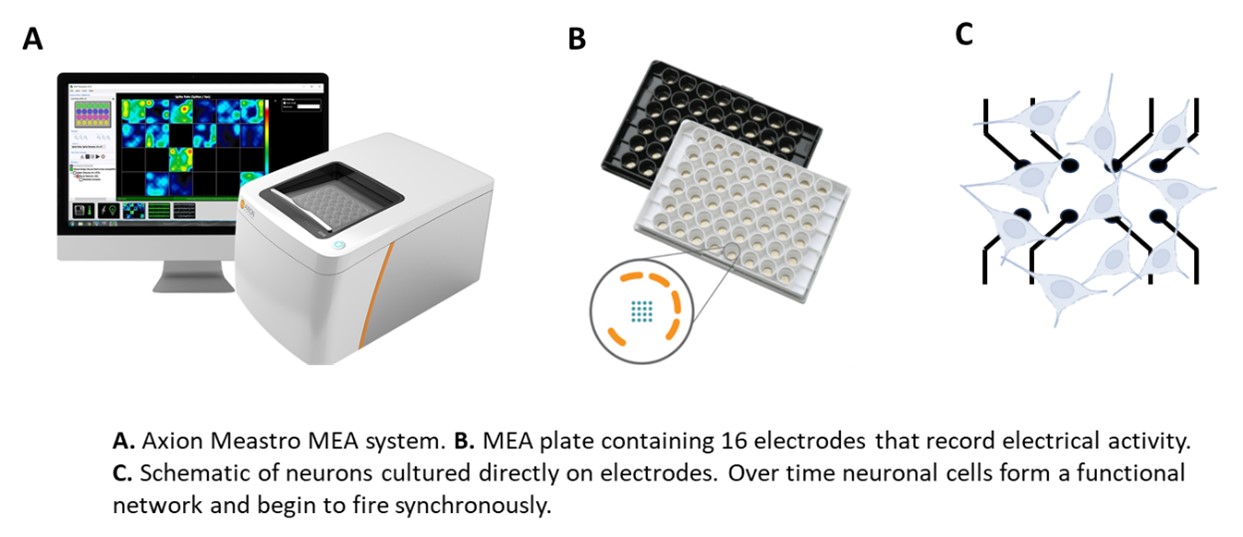
- A microelectrode array (MEA) assay that utilises human-derived neuronal stem cells to demonstrate potential seizure activity by measuring electrical activity
You will benefit from:
- High quality ion channel profiling with a turnaround time of 14 days from receipt of compound
- MEA assessment of electrical activity in human iPSC-neuronal and astrocyte co-cultures
- High-throughput assays with reduced reliance on costly animal studies with questionable translation
- Access to ApconiX scientists who will tailor our service specifically to your needs and better advise you on your next steps


Our Services:
- Ion channel screening of our comprehensive panel of seizure-associated ion channels performed by automated patch-clamp. All ion channels are the human isoform: Nav1.1, Nav1.2, Nav1.6, Kv1.1, Kv2.1, Kv3.1, Kv4.2, KCa1.1, KCa4.1, Kv7.2/7.3, Kv7.3/7.5, GABA α1β2γ2, NMDA 1/2A, nicotinic AChR α4β2, Cav2.1
- Investigation of potential seizurogenicity in hiPSC-neuronal co-cultures using the Maestro Pro MEA assay system (Axion BioSystems)
Investigating Seizure Liability
Inhibition of neuronal ion channels can adversely affect the balance of excitation and inhibition in the brain and negatively impact a drug’s probability of success, value and competitiveness. By working with ApconiX you will benefit from our combined expertise in ion channel electrophysiology, hiPSC-neuronal cell models and project toxicology to identify risks, gain mechanistic insight and prioritise the right candidates moving forward.
Our electrophysiology experts will rapidly generate high-quality seizure panel screening data for your drug discovery programme and aim to return this data in two weeks.
Our hiPSC-neuronal cell model experts can assess compound effects on electrical signalling. This can reveal perturbations to ion channel activity and other important regulators of neuronal function.