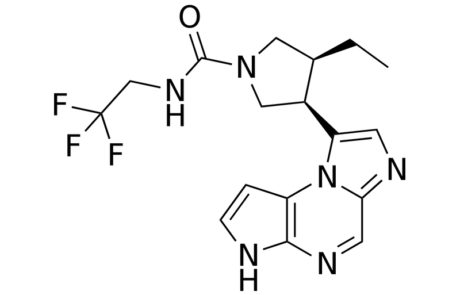
AbbVie’s Rinvoq label portends safety warnings for future JAKs- including Gilead’s
By Carly Helfand
An accumulation of safety liabilities including malignancy, thrombosis and serious infection are being reported from several previous and current clinical trials regarding JAK inhibitors such as fligotinib, xeljanz and olumiant.
Despite its recent approval due to an improved safety profile in comparison to other current JAK inhibitors, AbbVie’s Rinvoq has been branded with a black box warning as awareness of these safety implications increase and are believed to be class-wide regardless of pivotal trial data.
Sarepta Duchenne drug rejected by FDA in surprise setback
By Jonathan Gardner
Sarepta Therapeutics new drug, Vyondys 53, has been recently rejected by the FDA due to infection risk as well as pre-clinical indications of kidney toxicity. Therapeutic applications of Vyondys53 include treating a subset (8%) of Duchenne Muscular dystrophy patients by promoting dystrophin production by exon-skipping of exon 53. ( Exon 53 is missing in this subset of patients.) Following the approval of another Sarepta DMD drug in 2016 for a different subset of patients, the rejection of Vyondys 53 came as a surprise despite neither of them having “traditional placebo- controlled trials.”
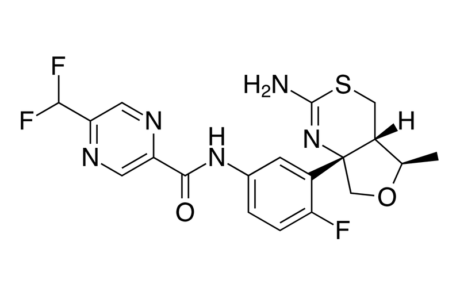
Biogen, Eisai throw out remaining Alzheimer’s BACE hope
By Ben Adams
After the failure of another amyloid targeting drug (aducanumab), Biogen and Eisai temporarily scrapped their BACE inhibitor drug, elenbecestat. This comes as the drug is deemed “unsafe” by the Data safety monitoring board who concluded “unfavourable risk-benefit ratio.”
One remaining phase III trial of Eisai’s BAN2401, may prove successful, although, the failure of now two Alzheimer’s Disease compounds in phase III trials, diminishes hopes for a cure in mild AD patients in the near future.
Amgen shares hit after analysts expose buried FDA trial halt
By Ben Adams
Progression of Amgen’s phase 1 oncology drug trial of AMG-397 is being held due to cardiac toxicity safety risks. AMG-397 is a small molecule, in trials to treat blood cancer by inhibiting MLC-1. It has since been found that MLC-1 may play a part in cardiac myocyte mitochondrial activity, hence causing a small number of cases of severe heart failure in patients involved in the dose escalation trial. Although only small number of cases, the significance of this adverse effect remains undetermined due to limited exposure of the drug. Despite this Amgen have also voluntarily halted trials of their drug AMG-176 as it too is an MLC-1 inhibitor.
Arbutus hit hard as now hepB drug fails on safety
By Phil Taylor
Arbutus reports that they are abandoning their Hepititis B drug AB- 506 based on clinical trial data indicating poor drug safety, particularly in two cases where healthy volunteers contracted acute hepatitis during treatment. This follows disappointing results and abandonment of RNAi ARB-1467 trial last December as well as delay of the RNA destabiliser AB-452 due to safety concerns.
Despite these set backs Arbutus claim to have other oral-capsid inhibitors in the pipeline with varying chemical structures to avoid the toxicity experienced with AB- 506.
Three breast cancer drugs: FDA warns of rare but serious lung inflammation
By Zachary Brennan
The FDA have approved new warnings for breast cancer drugs palbociclib, ribociclib and abemaciclib, due to new information regarding the risks associated with their drug classification. These drugs are generally classified as CDK4/6 inhibitors. Ongoing safety trials revealed interstitial lung disease (ILD) and pneumonitis (forms of lung inflammation) occurred in 1-3% of patients in treatment, despite many not having any prior risk factors. This proved fatal 1% cases. The FDA therefore advised pulmonary monitoring of a list of symptoms.
An Unexpected Halt in Multiple Myeloma for Venetoclax
By Derek Lowe
A clinical trial being run by Roche/Abbvie using ventroclax (ABT-199) has been unexpectedly halted due to a surprising 20% increase in patient mortality rate. Ventroclax’s unusually large molecular weight of 868 provides selectivity to Bcl-2 protein to induce apoptosis in lymphoma cells. Despite ABT-199 inducing kidney problems in earlier trials, it was pursued due to its efficacy against chronic lymphocytic leukaemia. The halted trial investigating it’s use against multiple myeloma in combination with other drugs, demonstrates varying expression of Blc subtypes and subsequent differences in drug action.

Newly Added Guidance Documents
By The FDA
The FDA regularly produce new guidance documents which are published to provide guidelines and advice to companies that are bringing new drugs to market.
The guidance help to ensure the most effective progression of a compound but also to consolidate compound safety.
The guidance documents are categorised for ease and can be searched in this way or by title or date. Particularly relevant categories for the work at ApconiX, drug safety and pharmacology/toxicology.
Common antibiotics may lead to condition that causes heart failure
By Tauren Dyson
The fluororquinolone class of antibiotic drugs have been linked to developing heart diseases and heart failure. Recent studies indicate a 2.4 fold increase risk of back flow of blood into the heart causing aortic mitral regurgitation and hence heart failure in patients taking fluoroquinolones apposed to other antibiotic drugs such as amoxicillin or azithromycin. Of >137,000 people, the study found that regurgitation was only common to recent or regular users of the drug and past users were statistically unaffected . This spurs wider research into the field and the safety of these classes of drugs.
