
UT Austin scientists design safer Cas9 with improved CRISPR gene editing accuracy
By Angus Liu
The CRISPR system is a powerful gene editing tool. Researchers have now refined Cas9, SuperFi-Cas9 which is much less likely to perform off target editing but is just as efficient at performing on target editing.
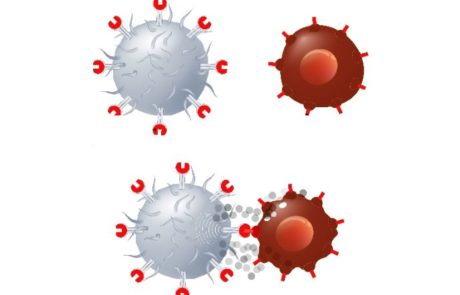
A review of cancer immuno therapy toxicity II: Adoptive cellular therapies, kinase inhibitors, monoclonal antibodies and oncolytic viruses
By N. Chhabra et al
Immunotherapy has undergone rapid expansion in classes agents and indications, though there is little clinical evidence of their adverse effects, mechanisms and toxicities.

Immune checkpoint inhibitors: Long term implications of toxicity
By D. Johnson et al
Immune checkpoint inhibitors are a new age of cancer therapeutics, providing improved survival of patients with significant metastatic disease. Evidence is building describing acute clinical toxicities of these agents. This paper reviews this evidence.

Use of whole slide imaging in nonclinical toxicology studies: Questions and Answers
By The FDA
In April 2022, the FDA released new draft guidance for industry surrounding the use of whole slide imaging. The documentation aims to clarify the FDA’s recommendations for management and use of whole slide imaging and inform sponsors and laboratories of GLP compliant human non-clinical toxicology studies.

Scientists home in on cause of Duchenne gene therapy side effect
By Jonathan Gardener
After a patient dies in the Pfizer Duchenne muscular dystrophy gene therapy trial, and other patients experienced symptoms of an immune response. Researchers now believe a genetic mutation may be responsible.

FDA warns of increased mortality with Duvelisib
By Mike Bassett
Duvelisib is a PI3 kinase inhibitor approved for use in adults with chronic lymphocytic leukaemia or small lymphocytic leukaemia. Recent results from the phase III DUO trial found that it has worse overall survival than other drugs.
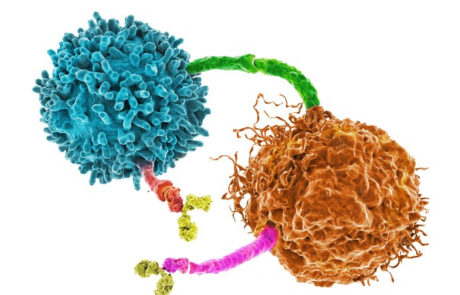
Are PD-1 and PD-L1 checkpoint inhibitors as good as we thought?
By Clara Rodriguez Fernandez
Interaction of PD-1 in T cells and PD-L1 in tumour cells is mechanism which tumours use to protect themselves from immune cells. Blocking this reaction would enable immune cell targeting of tumour cells. Unfortunately these checkpoint inhibitors do not work in all patients and require more work on their safety and efficacy.

Nonclinical considerations for mitigating nonhuman primate supply constraints arising from the COVID-19 pandemic
By The FDA
FDA is issuing this guidance to help sponsors mitigate the challenges related to the constrained supply of nonhuman primates (NHPs) available for conducting nonclinical toxicity assessments, which has arisen as a consequence of the current COVID-19 pandemic.

Clinical pharmacology considerations for the development of oligonucleotide therapeutics
By The FDA
This guidance provides recommendations to assist industry in the development of oligonucleotide therapeutics under section 505 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 355) and 21 CFR parts 312 and 314. Specifically, this guidance represents the FDA’s recommendations for certain evaluations including pharmacokinetic, pharmacodynamic, and safety assessments during oligonucleotide therapeutic development.
