An Integrated Approach for Early In Vitro Seizure Prediction Utilising hiPSC Neurons and Human Ion Channel Assays

ApconiX are delighted with our recent publication in Toxicological Sciences where we presented our new data on our integrated approach to detecting seizure. After 3 years of research, we launched the seizure liability assays earlier this year.
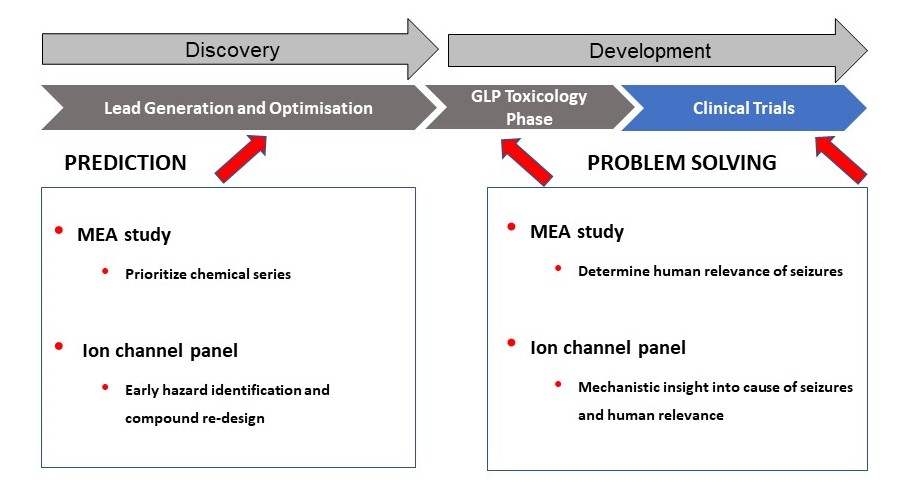
The combination of an ion channel panel linked to microelectrode array (MEA) offers a new paradigm in drug testing for seizure and was cited in a recent FDA/CDER paper (Avila et al., 2023), where the authors provide a perspective on the opportunities and challenges of using NAMs in drug development.
Professor Ruth Roberts commented, “What’s really interesting about the approach is that it is not necessarily a reduction, refinement or a replacement of an in vivo test but aims to change the landscape of seizure detection.”
Dr Kimberly Rockley added, “Since launching these assays we have helped many clients with seizure prediction and also helped to address problems in their drug projects caused by seizures seen both clinically and in preclinical species. Obviously, it would be far better to predict rather than resolve, so we are busy sharing this work with the drug discovery and development community.”
These studies highlight the potential utility of an integrated in vitro approach for early seizure prediction to provide mechanistic information and to support optimal drug design in early development, saving time and resources.
The Paper
Rockley K, Roberts R, Jennings H, Jones K, Davis M, Levesque P, Morton M. An integrated approach for early in vitro seizure prediction utilising hiPSC neurons and human ion channel assays. Toxicol Sci. 2023 Aug 26 https://doi.org/10.1093/toxsci/kfad087
Abstract
Seizure liability remains a significant cause of attrition throughout drug development. Advances in stem cell biology coupled with an increased understanding of the role of ion channels in seizure offer an opportunity for a new paradigm in screening. We assessed the activity of 15 pro-seizurogenic compounds (7 CNS active therapies, 4 GABA receptor antagonists and 4 other reported seizurogenic compounds) using automated electrophysiology against a panel of 14 ion channels (Nav1.1, Nav1.2, Nav1.6, Kv7.2/7.3, Kv7.3/7.5, Kv1.1, Kv4.2, KCa4.1, Kv2.1, Kv3.1, KCa1.1, GABA α1β2γ2, nicotinic α4β2, NMDA 1/2A). These were selected based on linkage to seizure in genetic/pharmacological studies. Fourteen compounds demonstrated at least one “hit” against the seizure panel and 11 compounds inhibited two or more ion channels. Next, we assessed the impact of the 15 compounds on electrical signalling using human induced pluripotent stem cell (hiPSC) neurons in microelectrode array (MEA). The CNS active therapies (amoxapine, bupropion, chlorpromazine, clozapine, diphenhydramine, paroxetine, quetiapine) all caused characteristic changes to electrical activity in key parameters indicative of seizure such as network burst frequency and duration. The GABA antagonist picrotoxin increased all parameters, but the antibiotics amoxicillin and enoxacin only showed minimal changes. Acetaminophen, included as a negative control, caused no changes in any of the parameters assessed. Overall, pro-seizurogenic compounds showed a distinct fingerprint in the ion channel/MEA panel. These studies highlight the potential utility of an integrated in vitro approach for early seizure prediction to provide mechanistic information and to support optimal drug design in early development, saving time and resources.





