Practical Application of Toxicology in Drug Development Course
Practical Application of Toxicology in Drug Development Course (PATDD)
Date: September 13th – 17th 2021
Location: This course will take place online. Pre-recorded lectures will be available prior to the live sessions.
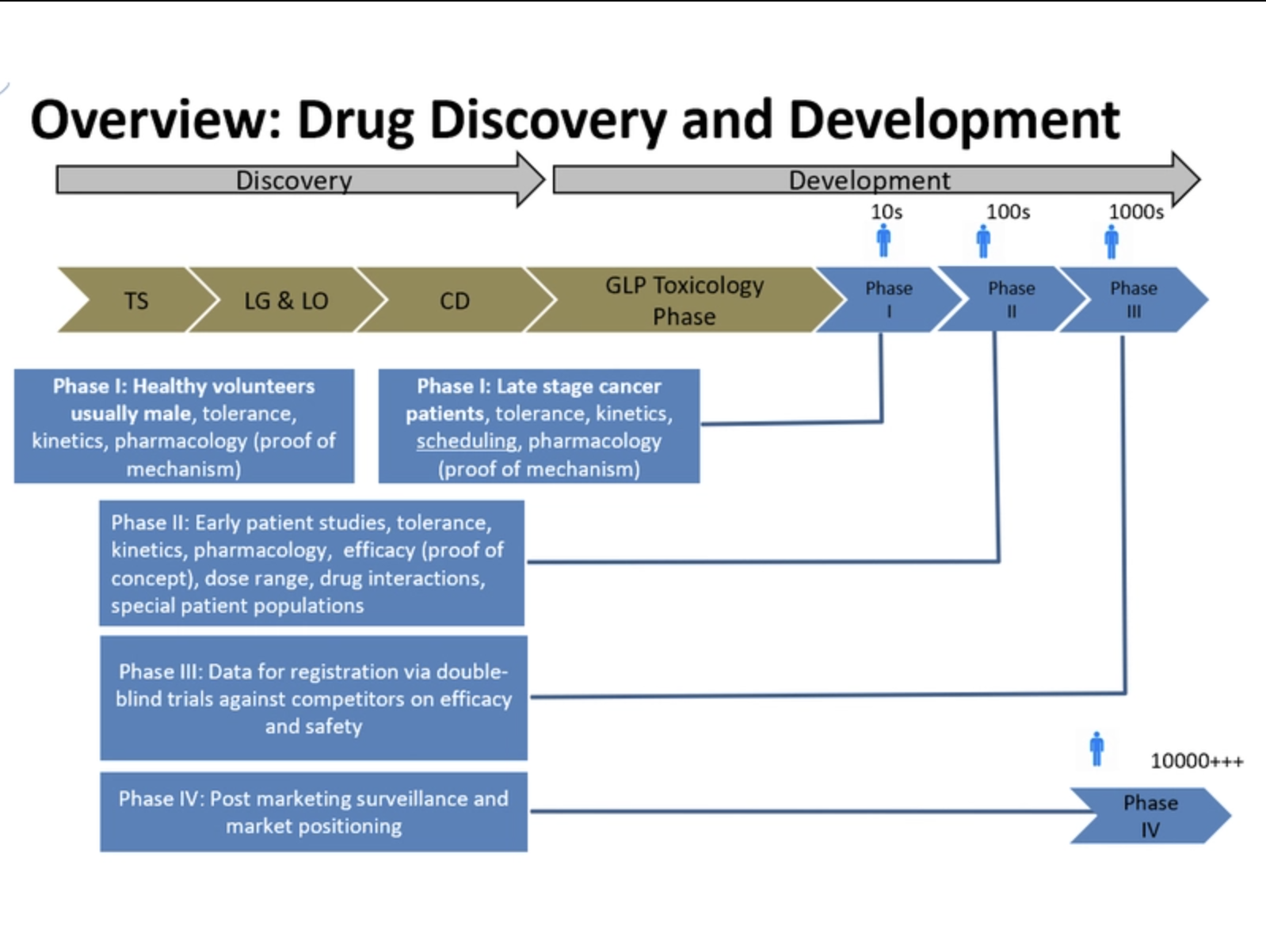
Overview of Drug Discovery and Development
On the first day, Ruth Roberts will be presenting an Overview of Drug Discovery and Development and will be joined by Rob Wallis, a Safety Pharmacology Consultant who will be discussing Pharmacology and Safety Pharmacology and Kunal Taskar, from GSK, who will be discussing DMPK and ADME, Concepts and Technologies.
About PATDD
This highly acclaimed course will provide training in toxicology as applied in drug development to scientists from all parts of the world. Participants will obtain an overall understanding of the principles of non-clinical safety evaluation with an emphasis on the practical application of these principles and the interpretation of non-clinical safety data. Regulatory toxicology in drug development will be emphasised, from both a European and a US perspective.
The course is intended to benefit those from both biotechnology and pharmaceutical companies as well as CROs working with either small or large molecules, along with those from regulatory agencies and academia who are interested in toxicology and its application in the safety assessment of drugs and medical products. Early career scientists seeking a more in-depth knowledge and understanding of the role of toxicology in safety assessment will also benefit. The course is suitable for scientists trained in ancillary disciplines (such as chemistry, biochemistry, molecular biology, medicine, etc.) looking to make a career change to work in drug safety assessment.
The course is recognised by EUROTOX as providing 37 hours of CPD and approved by the Royal Society of Biology for 96 CPD credits.
On-demand access to the presentations will be available to registrants for unlimited viewing no later than August 30th until September 25th 2021.
Virtual Features
• Virtual interactive case studies and break out groups
• Live Q&A Sessions with Speakers
• Recorded lectures and associated course materials with unlimited on-demand viewing
The course will take place from September 13th to 17th 2021 and will be held virtually. Pre-recorded lectures will be made available to registrants for unlimited viewing no later than August 30th 2021. Live Q&A sessions will be held with the speakers on the week of September 13th to 17th 2021 (Final 2-3 hour session times TBC) This annual course is a collaboration between the American College of Toxicology (ACT), the British Toxicology Society (BTS) and the Cambridge Alliance on Medicines Safety (CAMS).





