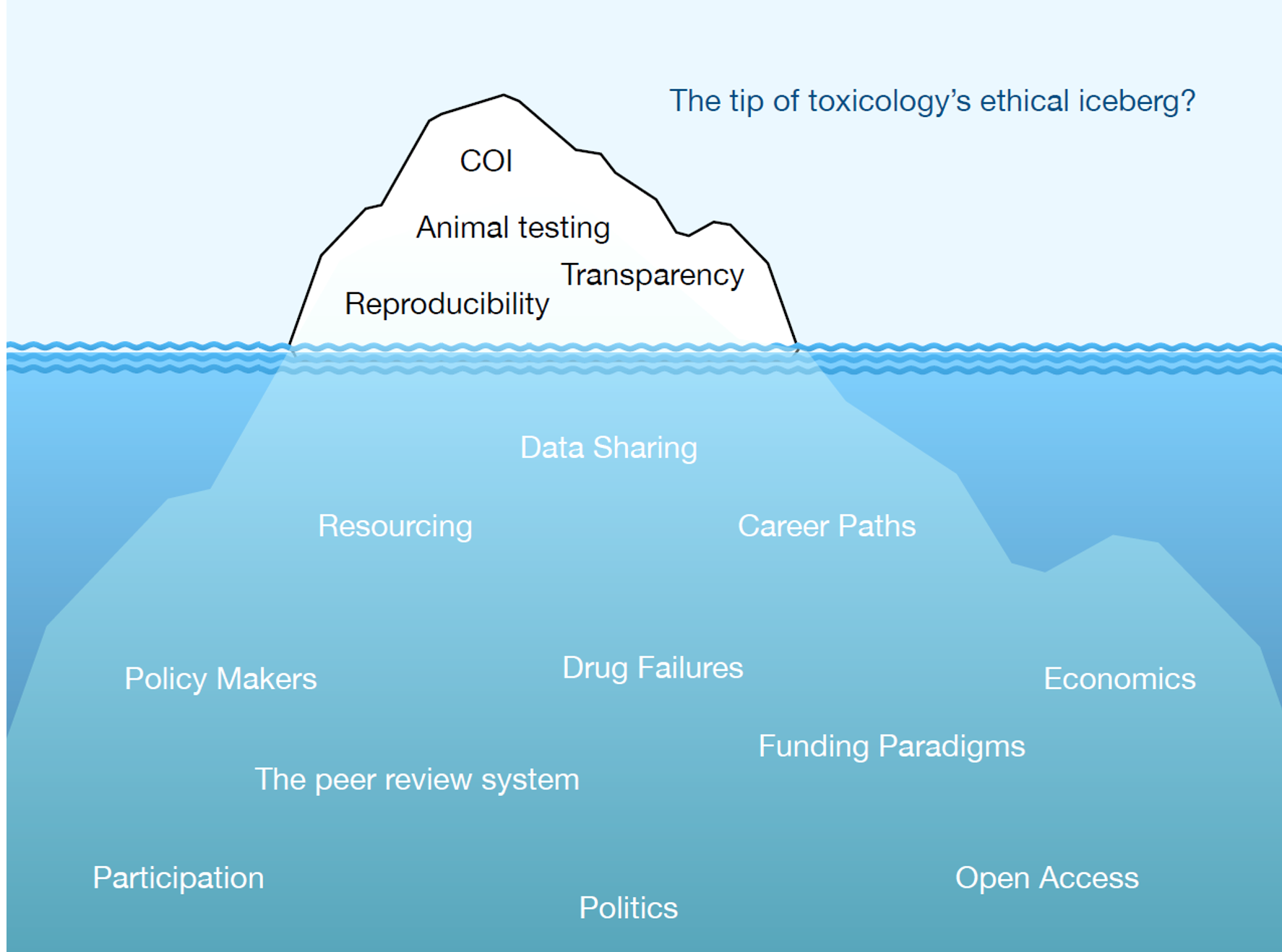
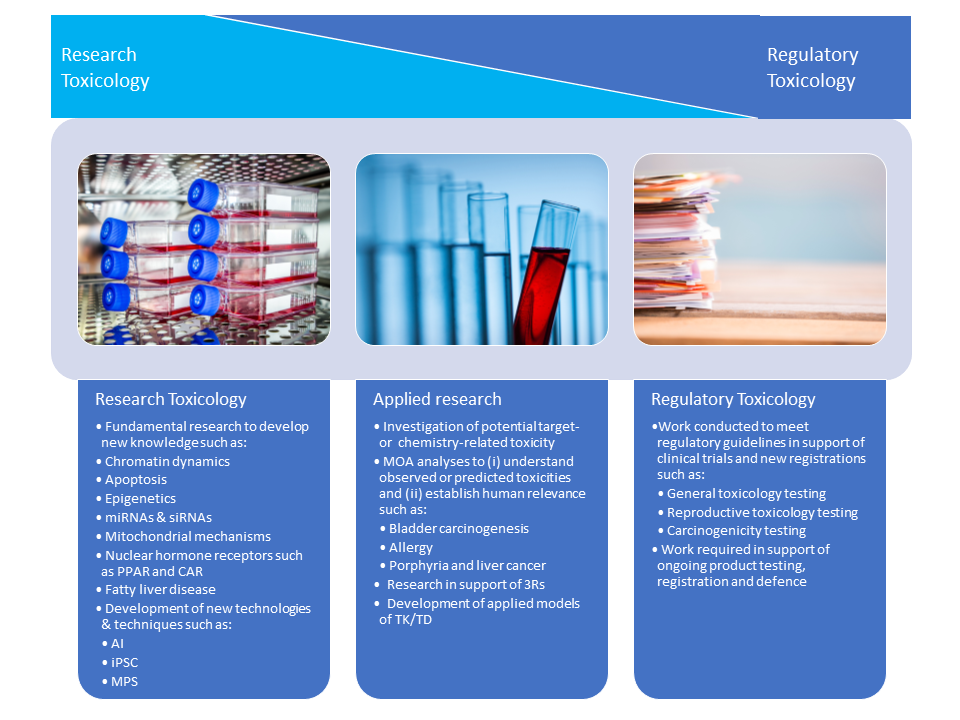
Two of the biggest ethical challenges in pharmaceutical toxicology are the use of animals in testing and the high safety-related attrition rates in new drug development. Yet very little is invested in these two fields compared with investment in new efficacy models, new disease targets and new technologies. How can this be addressed? In this article, we explore current paradigms in toxicology to address such issues as peer review, conflict of interest, resourcing, transparency and data sharing.
DOI: 10.1039/c7tx00306d
Click here for the pdf in press: Walker and Roberts ToxRes Feb 1st 2018