Toxicogenomics: A 2020 Vision
Together with the promotion of non-animal testing, in vitro toxicogenomics (TGx) may play a vital role in the next generation risk assessment paradigm. A strategic shift in risk assessment provides an unprecedented opportunity for repositioning TGx in the regulatory setting. As the emerging technique continues to impact the TGx field, novel genomic features such as miRNAs, ncRNAs, and circular RNAs may provide more resolution towards better understanding of the underlying mechanisms of toxicological processes. Advances in machine learning and artificial intelligence are gaining ground for their applicability in biomedical fields. In the near future, these advances may be further applied in the TGx field to improve predictive power.
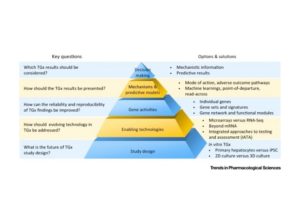
Toxicogenomics (TGx) has contributed significantly to toxicology and now has great potential to support moves towards animal-free approaches in regulatory decision making. Here, we discuss in vitro TGx systems and their potential impact on risk assessment. We raise awareness of the rapid advancement of genomics technologies, which generates novel genomics features essential for enhanced risk assessment. We specifically emphasize the importance of reproducibility in utilizing TGx in the regulatory setting. We also highlight the role of machine learning (particularly deep learning) in developing TGx-based predictive models. Lastly, we touch on the topics of how TGx approaches could facilitate adverse outcome pathways (AOP) development and enhance readacross strategies to further regulatory application. Finally, we summarize current efforts to develop TGx for risk assessment and set out remaining challenges.
Download the open access PDF: Zhichao Liu, Ruili Huang, Ruth Roberts, Weida Tong. Toxicogenomics: A 2020 Vision. Trends in Pharmacological Sciences, Vol. 40, Issue 2, p92–103