Our Posters
Building a predictive structure activity relationship (SAR) tool to mitigate ion channel related seizure liability in novel compounds
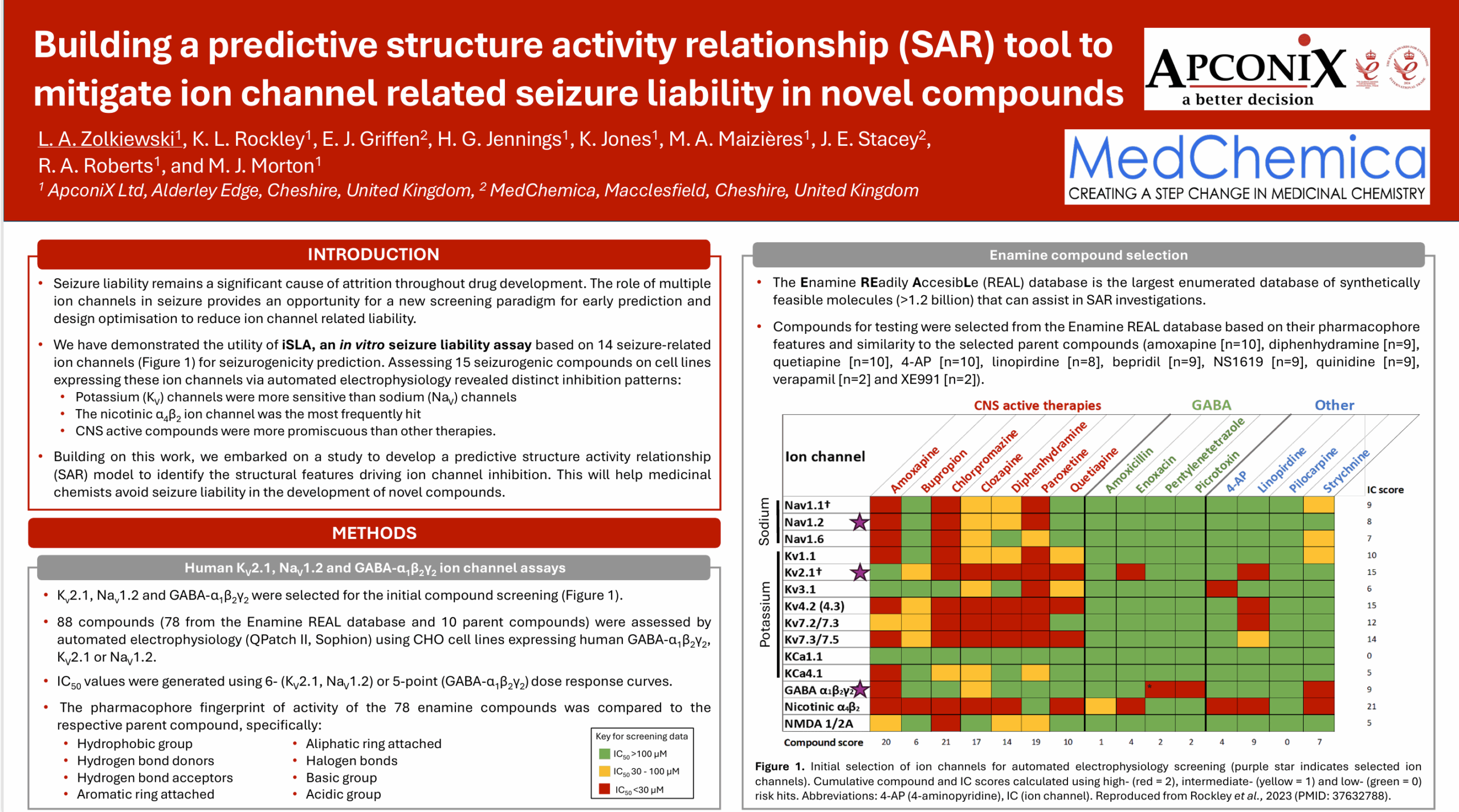
Background and Purpose: Seizure liability remains a significant cause of attrition throughout drug development. Human-based new approach methods (NAMs) are needed to provide an earlier prediction of seizurogenic risk to influence medicinal chemistry and reduce animal studies. We have previously demonstrated value in screening new drugs against numerous ion channels involved in seizure: Nav1.1, Nav1.2, Nav1.6, Kv7.2/7.3, Kv7.3/7.5, Kv1.1, Kv4.2, KCa4.1, Kv2.1, Kv3.1, KCa1.1, Cav2.1, GABA α1β2γ2, nicotinic α4β2, NMDA 1/2A. To assist in the development of therapies without seizure liability, we embarked on a study to develop SAR (structure activity relationship) by identifying the specific structural characteristics of the compounds that were associated with inhibition of the ion channels.
Methods: 88 compounds were selected from the Enamine REAL based on their pharmacophore features and similarity to the selected test compounds from the original screen (amoxapine, diphenhydramine, quetiapine, 4-AP, linopirdine, bepridil, NS1619, quinidine, verapamil, XE991). These compounds were screened using automated electrophysiology and the fingerprint of activity was compared to the parent compound for the Kv2.1 ion channel.
Results: The compounds tested show a range of activities and initial analysis shows some pharmacophores have a statistically significant higher potency. Individual pharmacophore dyads extracted show that distinct chemical groups may present a significant risk. This initial analysis provides evidence that some chemical groups may be more likely to inhibit Kv2.1, which may extend to other ion channels.
Conclusions: Future work testing the compound set across more ion channels will provide more evidence and help to build a SAR predictive tool. This will help structural chemists avoid seizure liability in the development of novel compounds.
Benefits of early in vitro screening for seizure liability in problem solving and decision making
Benefits of early in vitro screening for seizure liability in problem solving and decision making
Abstract (poster #P151)
K. Rockley, H. Jennings, E. Johnson, K. Jones, R. Roberts, M. Morton (ApconiX, Macclesfield, UK) pubmed.ncbi.nlm.nih.gov+8toxicology.org+8rscbmcs.org+8
Seizure liability remains a significant cause of attrition throughout drug development. In preclinical testing, seizures are among the most frequent CNS‑related adverse outcomes, encountered by up to 67% of respondents in a 2016 safety pharmacology survey. Between 2005–2010, CNS adverse effects were the most common reason for safety‑related attrition during clinical development.
Current methods primarily rely on rodent and non‑rodent animal studies, which can miss subtler seizurogenic risks and, if detected, often come too late—after substantial resource investment. There is a need for novel, high‑throughput in vitro methods to predict seizure risk earlier in discovery when medicinal chemistry can still modify structures or deprioritize compounds.
Building on the success of cardiac ion‑channel panels (e.g., CiPA) for arrhythmia risk, we developed an integrated in vitro seizure screening strategy comprising:
- A panel of 15 seizure‑related human ion channels (Nav1.1, Nav1.2, Nav1.6; Kv7.2/7.3, Kv7.3/7.5; Kv1.1, Kv4.2, KCa4.1, Kv2.1, Kv3.1, KCa1.1; Cav2.1; GABA α1β2γ2; nicotinic α4β2; NMDA 1/2A), selected via genetic and pharmacological linkage to seizure.
- A human iPSC‑derived neuronal microelectrode array (MEA) assay to detect seizurogenic electrical activity in cultured neuronal networks.
A set of 16 reference compounds—including known seizurogenic drugs (e.g. clozapine, diphenhydramine, bupropion, paroxetine, amoxapine) and acetaminophen as a negative control—was tested. All seizurogenic compounds produced at least one “hit” on the ion‑channel panel, and many inhibited two or more channels. MEA recordings revealed characteristic changes in network burst frequency and duration for CNS‑active compounds, whereas acetaminophen had no detectable effect.
Overall, pro‑seizurogenic compounds showed a distinctive electrophysiological fingerprint across the integrated ion channel/MEA platform. These results underscore the potential of this early in vitro screening approach to detect seizure liability—providing mechanistic insight, reducing reliance on animal studies, and enabling smarter decision‑making earlier in drug development.
Benefits of early in vitro screening for seizure liability in problem solving and decision making
Modulation of GABAA activity: Investigations in hiPSC-derived neuronal co-cultures and human ion channel assays
B. Kelly¹, K. Rockley¹, K. Jones¹, R. Roberts¹, M. Morton¹
¹ ApconiX, Alderley Edge, Cheshire, United Kingdom
Modulation of GABAA activity: Investigations in hiPSC-derived neuronal co-cultures and human ion channel assays
Biophysical and pharmacological properties of KV7 channels: an automated patch clamp study
Authors:
Kefan Yang¹, Fitzwilliam Seibertz¹, Markus Rapedius¹, Nina Brinkwirth¹, Michael Morton², Karen Jones², Nadine Becker¹, Sion Lee³, Alison Obergrussberger¹, Niels Fertig¹
¹ Nanion Technologies, Munich, Germany; ² ApconiX, Alderley Park, UK; ³ New Drug Development Center – KMEDIhub, Daegu City, Republic of Korea
Introduction:
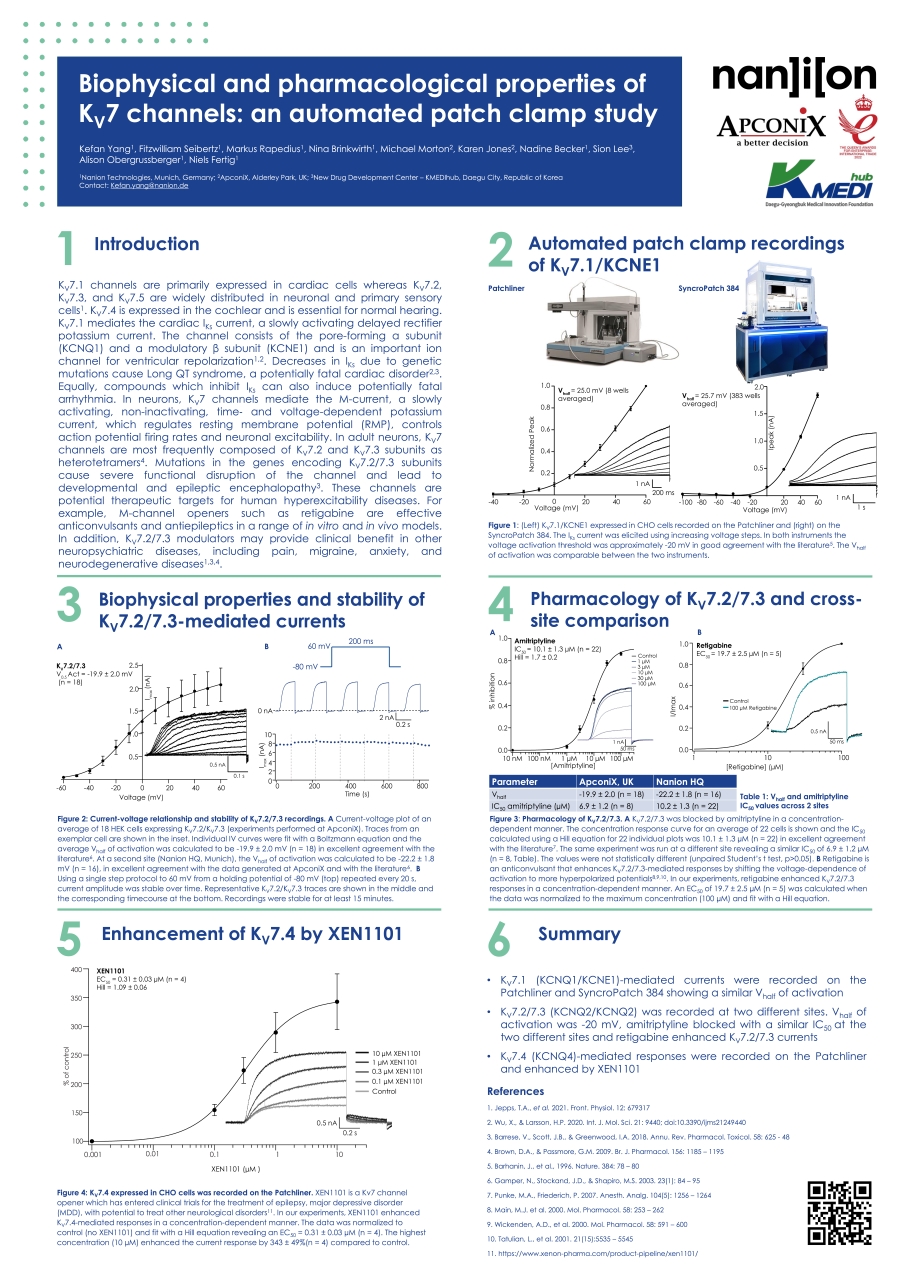
KV7.1 channels are primarily expressed in cardiac cells whereas KV7.2, KV7.3, and KV7.5 are widely distributed in neuronal and primary sensory cells. KV7.4 is expressed in the cochlear and is essential for normal hearing. KV7.1 mediates the cardiac IKs current, a slowly activating delayed rectifier potassium current. The channel consists of the pore-forming α subunit (KCNQ1) and a modulatory β subunit (KCNE1) and is an important ion channel for ventricular repolarization. Decreases in IKs due to genetic mutations cause Long QT syndrome, a potentially fatal cardiac disorder. Equally, compounds which inhibit IKs can also induce potentially fatal arrhythmia. In neurons, KV7 channels mediate the M-current, a slowly activating, non-inactivating, time- and voltage-dependent potassium current, which regulates resting membrane potential (RMP), controls action potential firing rates and neuronal excitability. In adult neurons, KV7 channels are most frequently composed of KV7.2 and KV7.3 subunits as heterotetramers. Mutations in the genes encoding KV7.2/7.3 subunits cause severe functional disruption of the channel and lead to developmental and epileptic encephalopathy. These channels are potential therapeutic targets for human hyperexcitability diseases. For example, M-channel openers such as retigabine are effective anticonvulsants and antiepileptics in a range of in vitro and in vivo models. In addition, KV7.2/7.3 modulators may provide clinical benefit in other neuropsychiatric diseases, including pain, migraine, anxiety, and neurodegenerative diseases.
Biophysical and pharmacological properties of KV7 channels: an automated patch clamp study

Impact of Target Expression on the Adverse Event Profile of Antibody-Drug Conjugates (ADCs): Implications for Target Safety Assessments
Authors:
Michelle Blissett, Will Humfrey, Nicholas Coltman, Sean Hammond, Helen Garside, James Sidaway, Ruth Roberts. ApconiX Ltd., Alderley Edge, United Kingdom.
Introduction:
• Antibody-drug conjugates (ADCs) are an emerging class of drugs comprised of three main
components: an antibody, a linker and a payload. Several antibody-drug conjugates (ADCs)
are already approved for use in oncology indications.
• The antibody target should have high expression in diseased cells and no or low expression
in normal tissues. However, expression of the antibody target in normal tissue may pose a
potential safety risk where cytotoxic payloads are utilized.
• The aim of this study was to guide target safety assessments (TSAs) by determining if target
expression data for ADCs correlates with reported clinical toxicity.
• TSAs use target biology, gene and protein expression data, genetic information from
humans and animals and competitor compound intelligence to understand and mitigate
the potential safety risks associated with modulating a biological target.
Impact of Target Expression on the Adverse Event Profile of Antibody-Drug Conjugates (ADCs): Implications for Target Safety Assessments

An integrated approach for early in vitro seizure prediction utilising human-derived induced pluripotent stem cells and human ion channel assays
Authors: K L Rockley, M Davis, H Jennings, K Jones, P Levesque, R A Roberts, M J Morton, ApconiX, Alderley Park, Alderley Edge, Cheshire, UK; Bristol Myers Squibb, Princeton Pike, New Jersey, USA
Introduction:
Seizure liability remains a significant cause of attrition throughout drug development. The seizurogenic potential of drug candidates is not typically evaluated until the late stage of preclinical discovery,
during in vivo toxicology studies. The timing of this assessment means that positive findings of seizure liability could result in the need to identify alternate clinical candidates. The resulting loss of
competitiveness, delays, increased costs, and considerable safety risk all emphasize the need for improved methodologies to detect seizure liability earlier, ideally with reduced reliance on costly animal
studies. High-throughput in vitro assays using human-derived induced pluripotent stem cell (hiPSC) neuronal cells coupled with screening seizure associated ion channels may offer an opportunity for a new
paradigm in screening. A combined approach could provide mechanistic insight into off-targets causative of seizure and improve identification of potential seizure risks preclinically.
An integrated approach for early in vitro seizure prediction utilising human-derived induced pluripotent stem cells and human ion channel assays

Improved Sustainability Through the Use of Assay Ready Cells to Support Ion Channel Screening
Authors:
H Jennings; K Rockley; K Jones; M A Maizieres; M J Morton
ApconiX Ltd, Alderley Park, Alderley Edge, Cheshire, UK.
Introduction:
Each year the consumables and equipment required for day-to day cell culture generates tons of plastic waste, which cannot be reused or recycled. This results in a significant impact to the environment and increases the cost of running a lab [1]. Our work in ion channel screening means we rely heavily on single use plastics which carry a financial and environmental cost and contribute to our carbon footprint. It has been estimated that each month our cell culture department generates an average of 80kg of consumable and plastic waste. In addition, recycling plants won’t accept lab waste due to contamination. One solution is to use assay ready cells in place of continuous cell culture. These cells are frozen and stored at high density and thawed immediately prior to assay, eliminating the need for prolonged culture. In this study we generated assay ready cell lines expressing a number of different ion channels and tested their performance on automated patch-clamp platforms. These cells showed excellent viability and functional activity, comparable to live cultured cells. In addition, the use of assay ready cells on automated patch-clamp systems has allowed us to streamline laboratory processes, halving the time it would usually take to generate client drug safety data. These steps have minimised the amount of plastic waste generated from cell culture by ~50% over the last 6 months. Extended testing is ongoing to confirm the reproducibility of these highly functional assay ready cell lines, and to implement these methods across all cell lines used at ApconiX.
Improved Sustainability Through the Use of Assay Ready Cells to Support Ion Channel Screening

Development of a human derived induced pluripotent stem cell neuronal assay for early in vitro detection of seizure liability
Authors:
K L Rockley1 ; R A Roberts1 ; M J Morton1. 1ApconiX, Alderley Park, Alderley Edge, Cheshire, UK
Introduction:
Seizure liability remains a significant cause of attrition throughout drug development. The resulting loss of competitiveness, delays, increased costs, and considerable safety risk all emphasise the need for improved methodologies to accurately detect seizure liability. A high throughput in vitro assay using human derived induced pluripotent stem cells (hiPSCs) to screen compounds for seizure liability may provide a solution with reduced reliance on costly animal studies. hiPSCs representative of the cellular subtypes present in the brain can be used to determine seizure risk in vitro using high-throughput micreoelectrode array (MEA). As with hiPSC-cardiomyocytes, batch-to-batch variability and the ratio of different cell types are important considerations.
Development of a human derived induced pluripotent stem cell neuronal assay for early in vitro detection of seizure liability

What are the common predicted toxicities from target safety assessments?
Authors:
J. Sidaway1, P. Sikakana1, B. Haynes2, H. Ge2 and R. Roberts1,2
1ApconiX Ltd., Alderley Edge, United Kingdom and 2University of Birmingham, United Kingdom
Introduction:
• Target safety assessments (TSAs) use target biology, gene and protein expression data, genetic
information from humans and animals, and competitor compound intelligence to understand the
potential safety risks associated with modulating a drug target.
• TSAs are used within drug projects to identify and mitigate risks, helping with informed decision
making and resource management.
• The aim of this work was to determine whether predicted toxicities from TSAs are consistent with
known drug safety insights (i.e. modality associations, mechanisms) and qualitatively similar to
toxicities observed during drug development. This was achieved by:
‒ Analysing the pattern of predicted toxicities from our TSA database based on drug modality,
mechanism of action (MoA), drug development status, drug target class, and therapeutic class.
‒ Evaluating whether predicted toxicities from our TSA database have a similar distribution and
characteristics to known toxicities described in published case studies.
What are the common predicted toxicities from target safety assessments?

Initial testing of an ion channel panel for early in vitro detection of seizure liability

A combined in vitro approach for early seizure prediction utilising human derived induced pluripotent stem cells and human ion channel assays
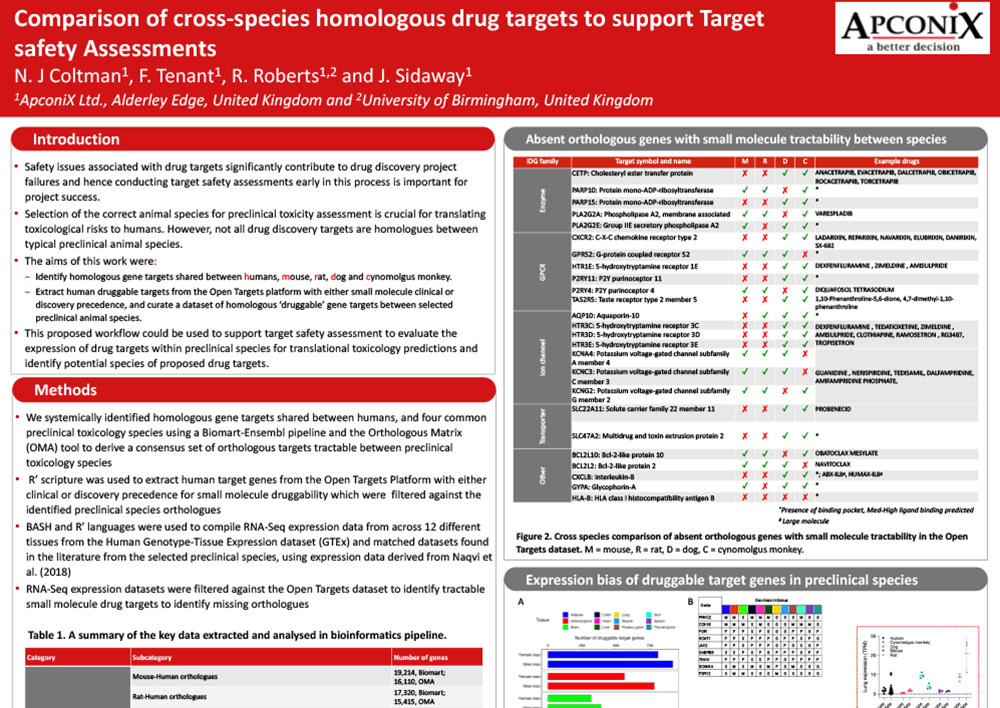
Comparison of cross-species homologous drug targets to support Target safety Assessments
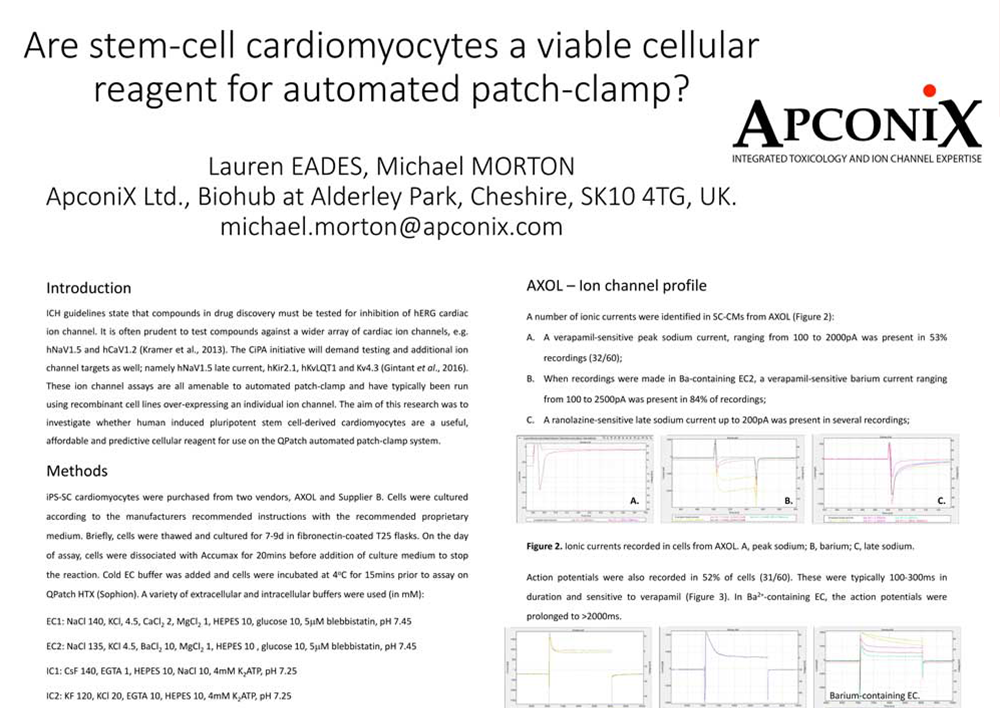
Are stem-cell cardiomyocytes a viable cellular reagent for automated patch-clamp?
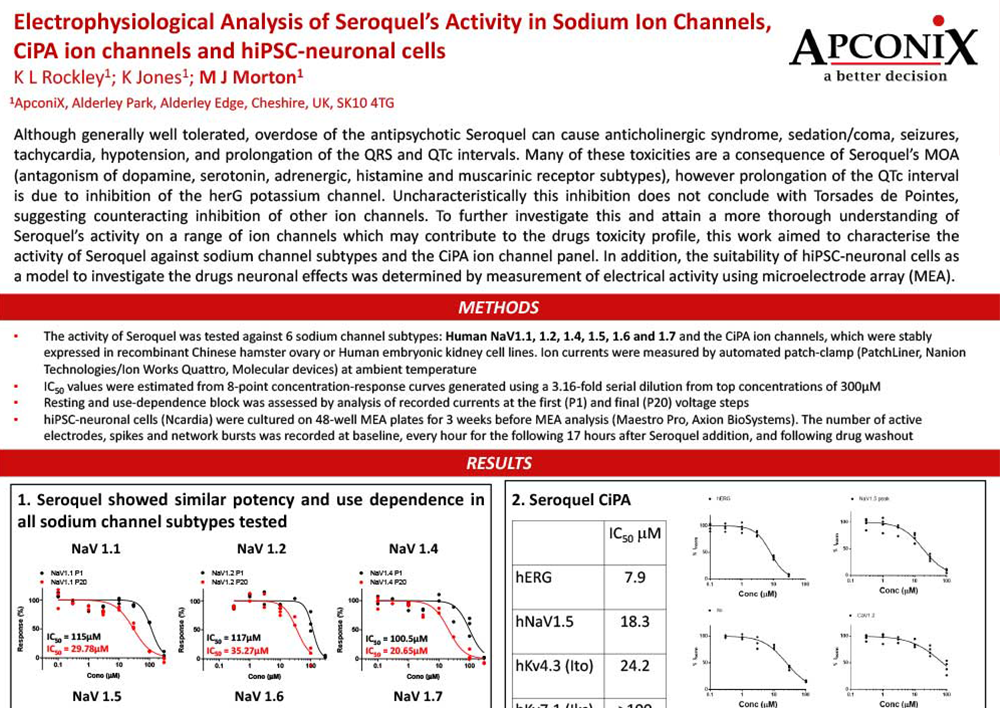
Electrophysiological Analysis of Seroquel’s Activity in Sodium Ion Channels CiPAion channels and hiPSC neuronal cells

Evaluation of the Toxicological Risk of targeting FRS (Phenylalanyl-tRNA Synthetase) in the Treatment of Malaria