Drug-induced liver injury severity and toxicity (DILIst): binary classification of 1279 drugs by human hepatotoxicity
Abstract:
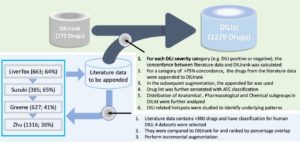
Drug-induced liver injury (DILI) is of significant concern to drug development and regulatory review because of the limited success with existing preclinical models. For developing alternative methods, a large drug list is needed with known DILI severity and toxicity. We augmented the DILIrank data set [annotated using US Food and Drug Administration (FDA) drug labeling)] with four literature datasets (N>350 drugs) to generate the largest drug list with DILI classification, called DILIst (DILI severity and toxicity). DILIst comprises 1279 drugs, of which 768 were DILI positives (increase of 65% from DILIrank), whereas 511 were DILI negatives (increase of 65%). The investigation of DILI positive–negative distribution across various therapeutic categories revealed the most and least frequent DILI categories. Thus, we consider DILIst to be an invaluable resource for the community to improve DILI research.
Read the open access paper here: Shraddha Thakkar1 Ting Li1, Zhichao Liu1 Leihong Wu1, Ruth Roberts23, Weida Tong1, Drug-induced liver injury severity and toxicity (DILIst): binary classification of 1279 drugs by human hepatotoxicity. Drug Discovery Today Volume 25, Issue 1, January 2020, Pages 201-208.
1Division of Bioinformatics and Biostatistics, National Center for Toxicological Research, US Food and Drug Administration, Jefferson, AR 72079, USA
2ApconiX Ltd, Alderley Park, Alderley Edge, SK10 4TG, UK
3University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK