An Ascorbate-Induced Increase in Sodium Current
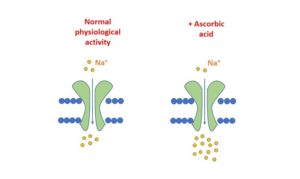
The Challenge
It is not uncommon for our clients to synthesise compounds for testing in in vitro assays that are largely insoluble, and sometimes this requires consideration of novel vehicles. Recently, when provided with a DMSO-insoluble compound, we explored using the aqueous solvent, ascorbic acid.
The Science
Firstly, it was important to examine any potential effects of ascorbate itself on ion channel activity in our automated electrophysiology assays. Using Patchliner (Nanion Technologies) with CHO cells expressing human cardiac sodium channel hNaV1.5, we explored channel activity in the presence of L-ascorbic acid (Sigma # 0278). Buffer pH was adjusted as necessary.
The experiment measured sodium current against increasing concentrations of ascorbate, up to a concentration of 1%. The results showed that sodium current increased in a concentration-dependent manner with increasing ascorbate concentration, as shown in Figure 1.
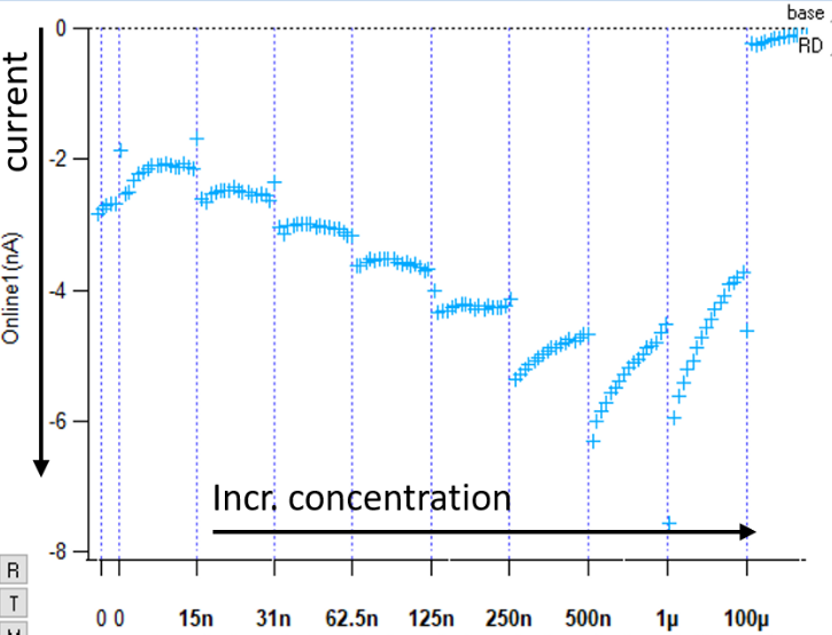
Figure 1: Current produced through hNaV1.5 increases as concentration of ascorbate increases. Tetracaine used as a control compound to demonstrate a channel block.
In the literature there is currently no clear explanation for this phenomenon of an ascorbate-induced increase in sodium current. 1% ascorbate does create a hypertonic solution, so could this effect be due to an increase in osmolarity that helps drive ionic currents? Whatever the explanation, this work underlines the importance of thorough consideration of potential vehicle effects prior to the use of uncommon solvents in these assays. Any intrinsic activity of the vehicle itself might serve to exacerbate or diminish the activity of the test compound.
ApconiX has also looked at a number of other solvents used to improve compound solubility or stability, for their potential effects on ionic currents. Methanol and isopropanol could be used at concentrations up to 1% without effect in standard CiPA assays. In contrast, Tween, which is often used to stabilise proteins/peptides, was found to have effects even at concentrations as low as 0.001%.





