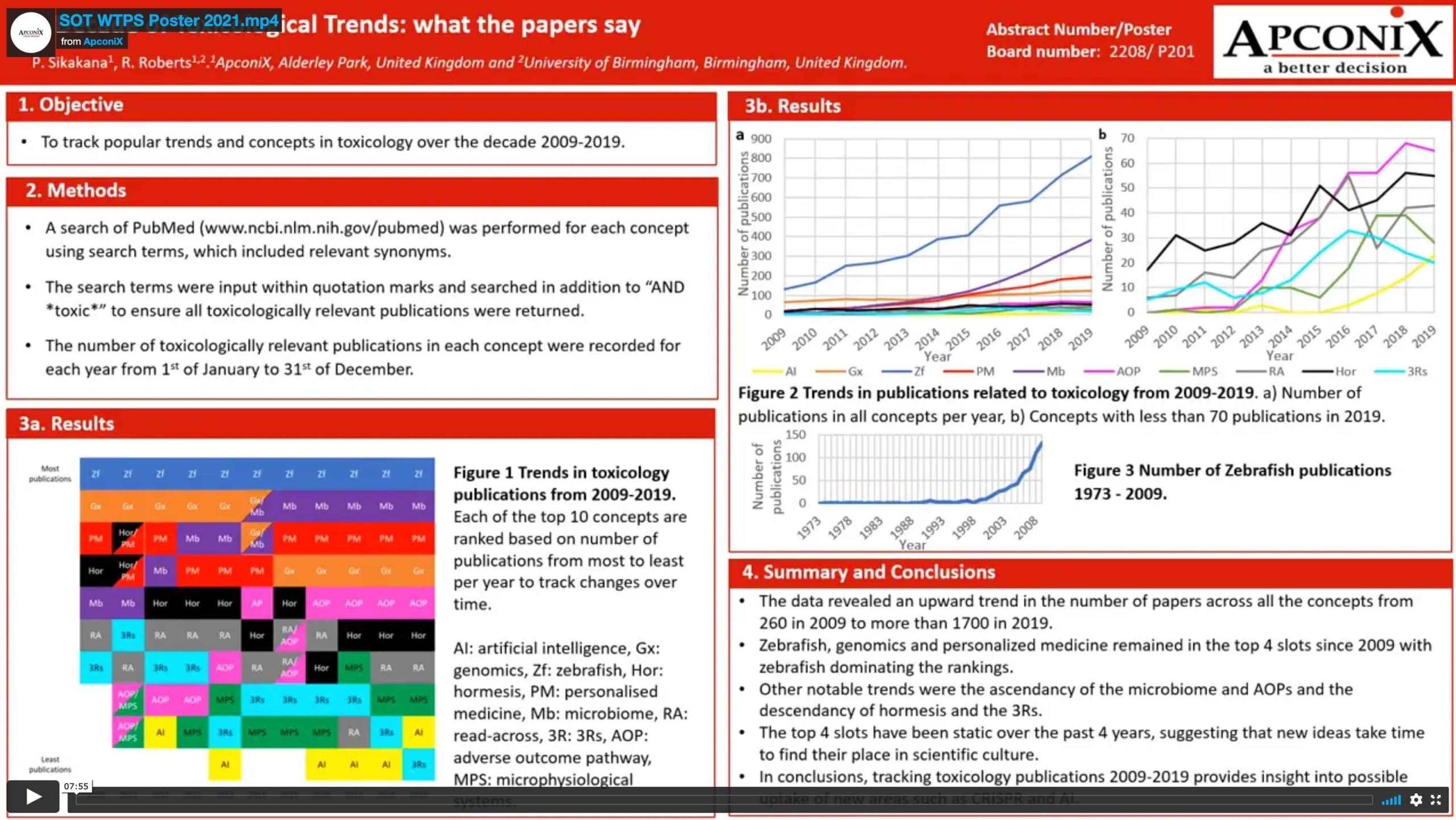
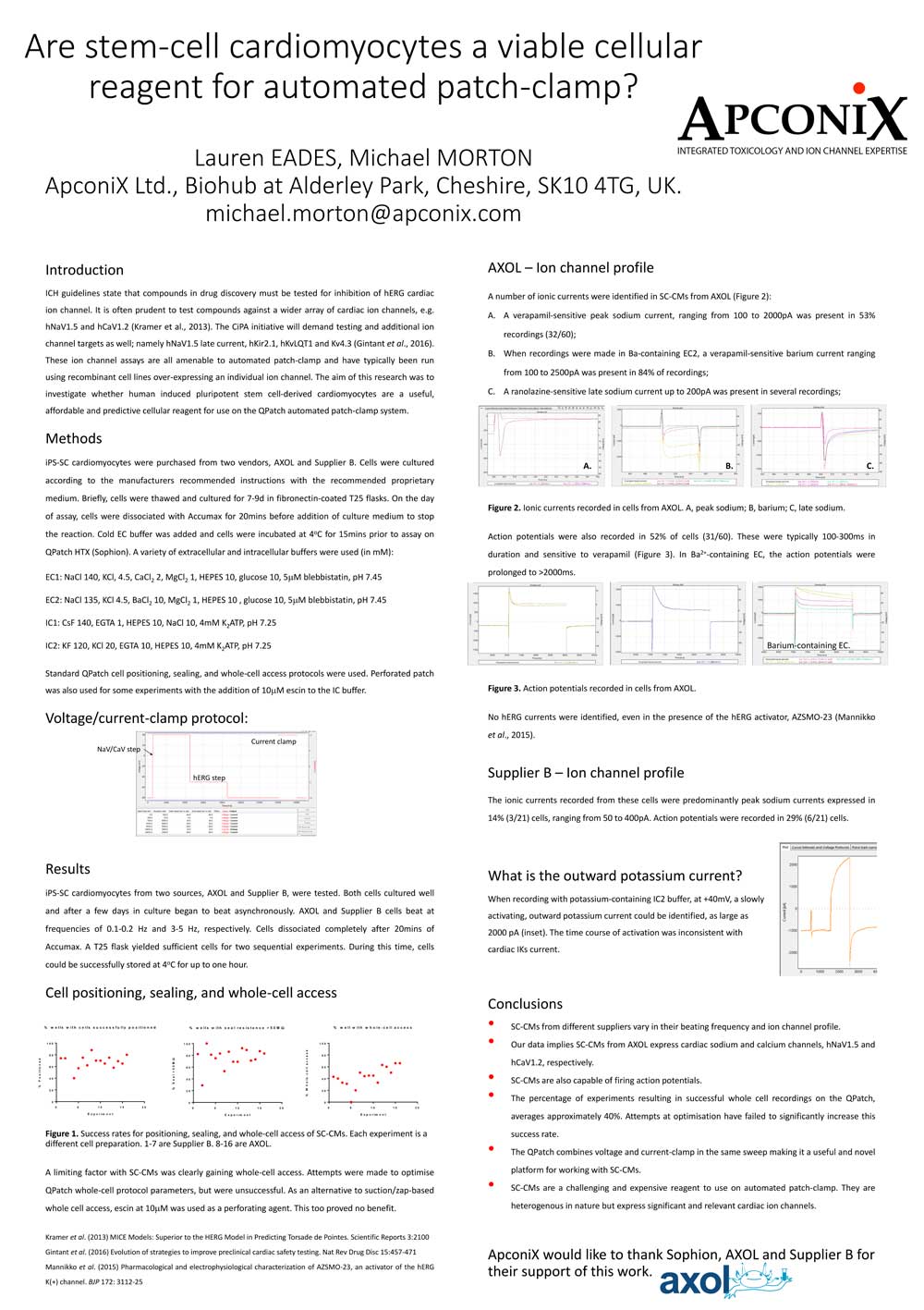
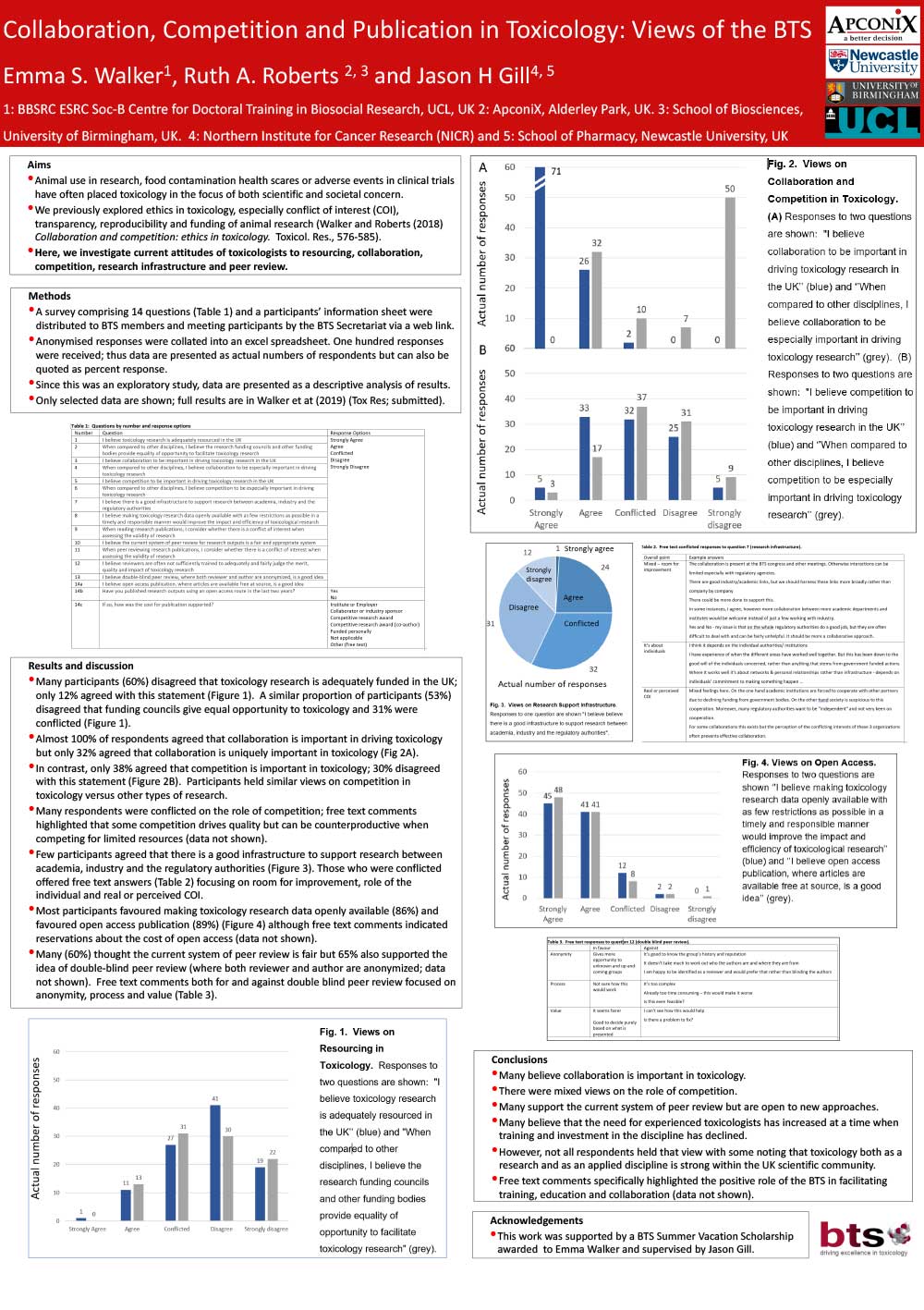
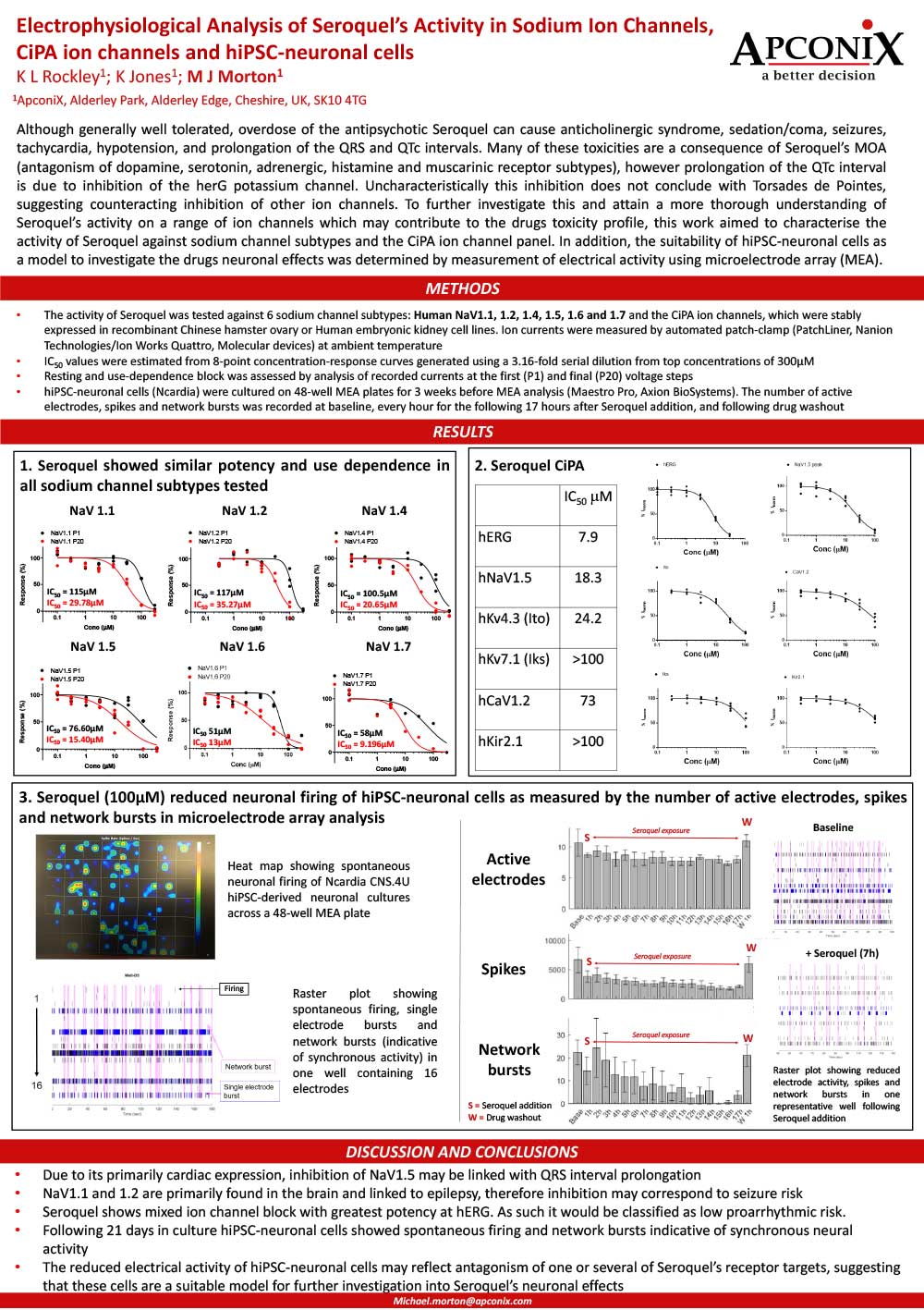
Download our posters resources here
Welcome to Apconix’s Resources page – click on the button below to download the resource.
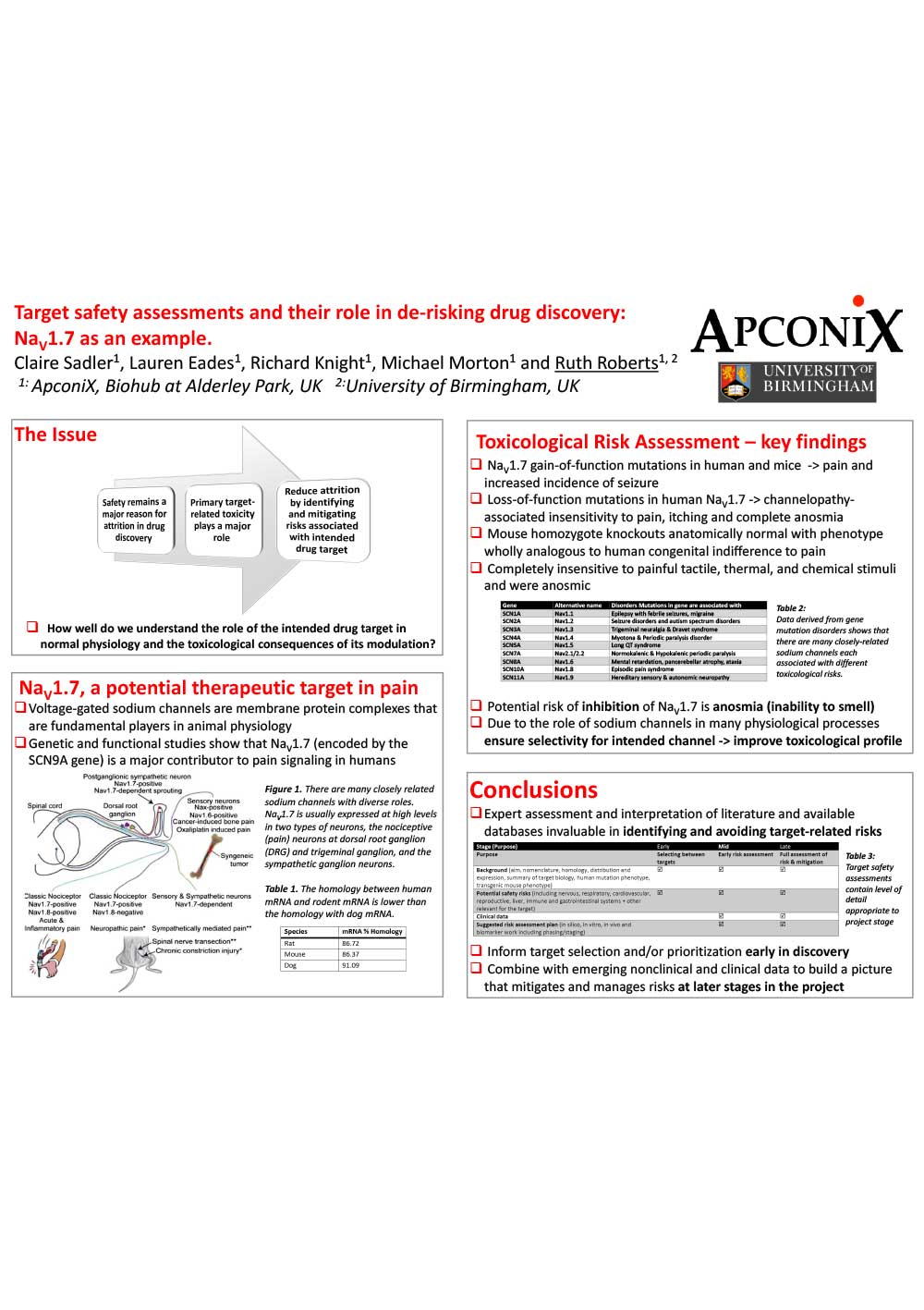
Target safety assessments and their role in de-risking drug discovery NaV1.7 as an example

The CiPA Profile of Two Adenosine Uptake Inhibitors dilazep and dipyridamole